Beauty Breakdown – Cleanse

Cleansers exist in diverse forms – gels, creams, oils, balms and even waters.
Despite their apparent differences, have you ever wondered how they are able to do the same job?
Why use cleansers
Cleansing gets rid of oil residues (e.g. makeup, excess sebum, environmental pollutants, skin care products) from skin. As oil is not fully removable by water alone, we need cleansers because they contain surfactants (surface active agents), which
- lift off the unwanted oils
- remove them from skin surface
This is why pure oils alone (coconut, olive etc) cannot clean properly – while they can lift makeup off easily, they cannot be fully washed away from skin with only water.
So, how exactly do surfactants work?
The science of surfactants
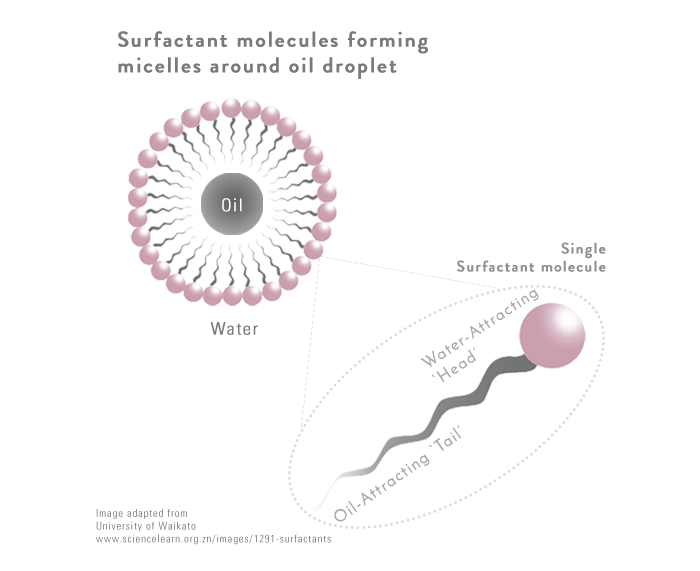
Every surfactant molecule has a head that attracts water and a tail at the opposite end that attracts oil.
This allows them to
- Bind oil impurities on skin with their oil-attracting tails
- Move towards water particles (when water is added) due to their water-attracting heads, and finally
- Wash away, with the attached impurities, from skin surface
FUN FACT: When multiple surfactant molecules are present, they form circular structures called micelles, where all their water-attracting ends stick to one another. So actually, all cleansers contain micelles, they are not just in micellar waters!
Cleansers can come in any shape or form as long as they contain surfactants, which are essentially what removes the oil impurities from skin.
Want to know more about which surfactant cleanses better? We’ll be looking into the different types of surfactants next. Stay tuned!